Abstract
Acquisition of multiple somatic mutations and epigenetic changes can result in malignant transformation, intra-tumoral heterogeneity, therapy resistance and disease relapse. However, our current knowledge of how cooperating driver genetic and epigenetic events shape gene regulation and chromatin landscapes in patient samples is still limited.
To address this, we developed Genotyping with the Assay for Transposase-Accessible Chromatin (GTAC), a novel method for high efficiency capture of open chromatin with parallel amplification of multiple genomic targets. This provides genetic clonal identity and epigenetic state at single-cell resolution (Fig. 1). We first tested this method in leukemia cell lines. GTAC successfully detected 8 genomic loci encoding leukemic variants in 96-100% of single cells. Low allelic dropout rates (1.5% - 11%) allowed high-confidence mutation calling for heterozygous loci in 90-99% of cells. Corresponding scATAC-seq libraries were of high quality, independent of the number of genotyped loci, with >105 unique fragments/cell on average and unperturbed signal-to-noise ratio. 93.75% of cells had simultaneous detection of all 8 genomic targets and efficient scATAC-seq library generation. Finally, a mixing experiment on three cell lines showed that 97%, 96% and 99% of cells had correct mutation calls for the ASXL1, RUNX1 and NRAS loci, respectively.
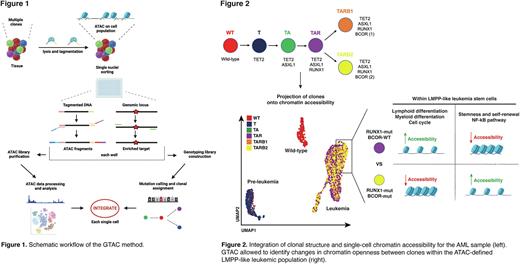
We next applied GTAC to 3520 single cells from a primary human acute myeloid leukemia (AML) sample harbouring mutations in TET2, ASXL1, RUNX1, and two BCOR loci (Fig. 2). Single-cell genotyping successfully assigned clonal state for 96.8% of cells. This showed that TET2, ASXL1, and RUNX1 mutations were acquired sequentially, followed by branched acquisition of two independent clones with BCOR loss-of-function mutations. Integration of clonal structure and chromatin accessibility revealed that wild type cells were mainly found in T/NK cell clusters, while TET2 and TET2/ASXL1 mutant cells were predominant in the hemopoietic stem and multipotent progenitor clusters, which were most likely preleukemic. In contrast, on acquisition of the RUNX1 mutation, cells were entirely distributed within lympho-myeloid progenitor-like compartments, establishing the importance of the RUNX1 mutation in transformation from preleukemia to leukemia. Global transcription factor binding motif analysis confirmed profound chromatin landscape changes between clones. RUNX1 mutant cells were strongly enriched in motifs of pro-proliferative transcription factors and those central to lympho-myeloid differentiation, such as SPI1, CEBPA, BCL11A/B and FOS/JUN.
Further clonal evolution occurred within leukemic cells, resulting in a significant enrichment of BCOR mutant clones within the lymphoid-primed multipotent progenitor (LMPP)-like differentiation state (P<0.0001). Interestingly, this cluster was characterized by higher accessibility of stemness-related HOXA9, MECOM, HLF, FOXO, ERG and ETV6 binding motifs coupled with higher CD34 and lower CD38 accessibility. LMPP-like cells also displayed higher accessibility of leukemia stem cell (LSC)-associated genes (Gentles et al., JAMA, 2010). Moreover, BCOR mutant cells were enriched in MEF2 family motifs linked to AML therapy resistance. This, together with our previous data showing LSC function within LMPP-like cells, suggests a bias towards an LSC-like chromatin state upon BCOR loss.
Our dataset uniquely allowed us to examine the impact that BCOR loss-of-function exerts on chromatin accessibility specifically within LMPP-like cells by comparing ATAC profiles of BCOR-mutant and wild type cells within this compartment. BCOR mutant LMPP-like cells had increased accessibility of motifs bound by HOXA/B, NANOG, and NF-kB transcription factors, and a more open INPP4B gene, recently associated to LSC maintenance (Woolley et al., BioRxiv, 2022). This suggests that aberrant PRC1.1 activity, caused by BCOR gene loss, may further confer a fitness advantage to BCOR mutant cells in the TET2/ASXL1/RUNX1-mutant context.
In summary, GTAC enables detailed single-cell analysis of chromatin landscapes in normal, pre-malignant and fully transformed subclones within complex clonal hierarchies in primary patient cells, revealing how gene regulation is corrupted as clones evolve. GTAC is a widely applicable tool to study malignant and pre-malignant states.
Disclosures
Davies:Nucleome: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company. Hughes:Nucleome: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Vyas:Celgene: Honoraria, Research Funding; Abbvie: Honoraria; Astellas: Honoraria; Daiichi Sankyo: Honoraria; JAZZ: Honoraria; Pfizer: Honoraria; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal